Abstract
Introduction - IMIDs are critical in the treatment of multiple myeloma (MM). Unfortunately, many patients become resistant and this is a major challenge in MM therapy. IMID resistance can be caused by CRBN mutations (rarely) and by upregulation of specific antioxidative mechanisms. We hypothesized that measuring baseline antioxidative capacity might be a useful predictor of IMID sensitivity in the clinic.
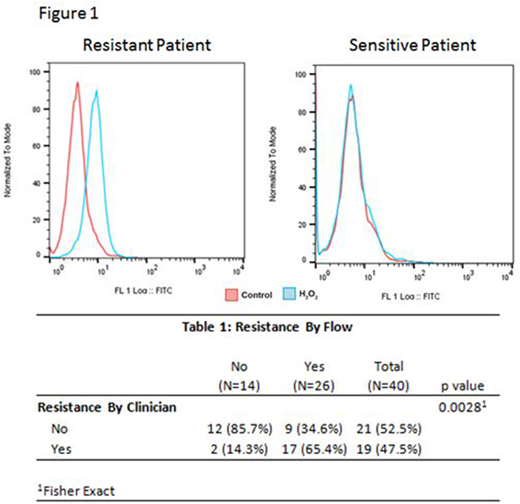
Methods - To develop a quantitative assay for determining cellular antioxidative capacity of MM cells we measured total cellular oxidized flavin adenine dinucleotide (FAD) after treatment with H2O2. FAD has auto-fluorescent properties that can be measured via flow cytometry. Cells with high antioxidative capacity generate more FAD, and cells with low anti-oxidative capacity generate less FAD. MM cells with an increase in FAD after H2O2 were considered IMID resistant and those with no change in FAD were considered IMID sensitive (Figure 1). Clinical IMID resistance/sensitivity was defined clinically during blinded retrospective review, with disease progression based on the IMWG criteria. After blinded review of clinical outcomes, the patient flow cytometry results were disclosed to evaluate the predictive value of the flow cytometry assay for detection of IMID resistance.
Results - Of 40 patients who underwent testing, 26 were IMID resistant via flow and were considered as being clinical resistant 17 (65%). Conversely, 14 patients were IMID sensitive via flow and 12 (86%) were considered to be clinical sensitive. There was a significant correlation between clinical assessment and flow cytometry in determination of IMID resistance patterns (Table 1). The sensitivity and specificity of our assay was 89% and 57% with a positive and negative predictive value of 65% and 86% respectively. A limitation of our clinical assessment was that 16 patients (38%) were treated with combination therapy; IMIDs plus a proteasome inhibitor or a monoclonal antibody. Of the 11 patients with discordant results by flow and clinical outcome, 7 (64%) were not on therapy at the time of testing and 6 (55%) had flow testing on the bone marrow sample performed on day 100 following an autologous transplant. Two (7%) of the 29 patients with correlative results were off all therapy at the time of testing, 1 with testing on the day 100 marrow sample after transplant.
Conclusions - This early testing of our assay shows promising results predicting clinical sensitivity via our flow based assay. The testing demonstrates a high sensitivity and negative predictive value, with less ability to assess for resistance. However, there were limitations to this study. The majority of patients with discordant results were off IMID therapy at the time of flow cytometry and many of these were assessed on transplant day 100 marrow samples. Depth of measureable residual disease was not readily available on all marrow samples tested. It is probable that flow testing for IMID resistance is inaccurate in patients with deeper responses to therapy, especially those who are minimum residual disease (MRD) negative. Additionally, several patients were on combination IMID, proteasome inhibitor, and monoclonal antibody. It is possible that clinical IMID resistance was not detected due to the anti-MM activity of the additional therapies. Additional larger scale prospective studies are needed to validate these findings as this method of testing could potentially provide providers with powerful information allowing them to personalize therapy for their patients.
Fonseca:Mayo Clinic: Employment; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal